Recently, Prof.Chang Lingqian from School of Biomedical Engineering, AHMU published his research entitledA finger-driven disposable micro-platform based on isothermal amplification for the application of multiplexed and point-of-care diagnosis of tuberculosisinBiosensors&Bioelectronics (IF=10.7). Partnering with researchers from Beihang University, Prof. Chang and his team introduced a portable finger-driven microfluidic chip (named Fd-MC) with the cost below 8 dollars, which could be ideal for large-scale on-site rapid tuberculosis(TB) detection in areas short ofmedical resources. Together with Prof. Ying Binwu from Department of Laboratory Medicine at West China Hospital, Prof. Chang served as the corresponding author of this paper.
Mycobacterium tuberculosis(MTBC) is responsible for the potentially major pandemic of TB which causes high mortality rate in patients. Prof. Chang said, “Clinical TB screening and diagnosis are so far mainly conducted on limited types of commercial platforms, which are expensive and require skilled personnel,thus usually only available at well-equipped tertiary hospitals. As a result, clinics and hospitals in remote regions are in need of a new test platform.”
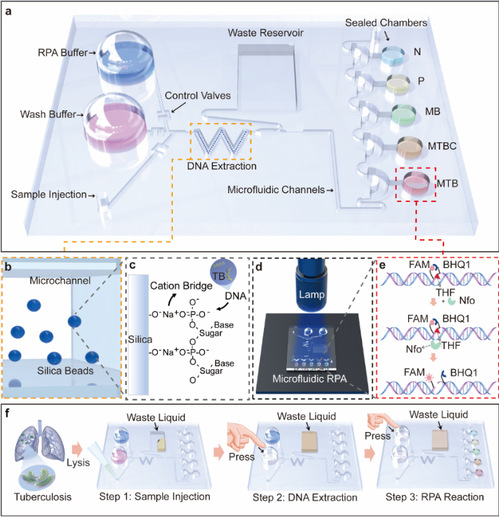
To meet this demand, Prof. Chang and his team designed this low-cost portable Fd-MC based on recombinase polymerase amplification (RPA) technique, which enables nucleic acid extraction, amplification and testing of the MTBC in the sputum samples. The device consists of a laser-engraved PMMA substrate, and a buffer-storage area covered by polydimethylsiloxane which, under a gentle press, deforms and drives the buffers to downstream reaction zones through each micro-channel. In doing so, the scientists managed to create an electricity-free paradigm and eliminate the need of external pumps. With this chip, Isothermal amplification within 20 minutes can be effectuated within the temperature range between37℃and 42℃.
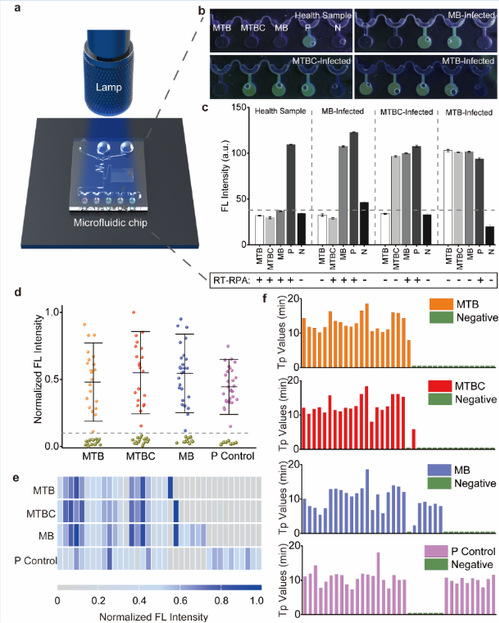
The platform was verified in 37 clinical samples, statistically with 100%specificity and 95.2% sensitivity as compared to commercial real-time RPA. Moreover, designed for multiplexed testing, the chip incorporates an in-situ fluorescence strategy based on FAM probe which allows for on-chip results read-out though a hand-held UV lamp, eliminating the risk of cross-contamination.
The full text of this paper is available at https://www.sciencedirect.com/science/article/abs/pii/S0956566321007004
