Liu Qi, an associate professor of AHMU, and Liu Yang, a researcher of the School of Chemistry of Nankai University published a paper entitled “Removing Tumor Immunogenicity with Dual-Activatable Binary CRISPR Nanomedicine for Cancer immunotherapy” on ACS Nano, a top international journal in the field of nanoscience (CAS Division I, IF=18.027). The corresponding authors of this paper are Liu Qi, Liu Feilong, associate professors of the School of Pharmacy of AHMU, and Liu Yang, a researcher of Nankai University, School of Pharmacy of AHMU being the first affiliation.
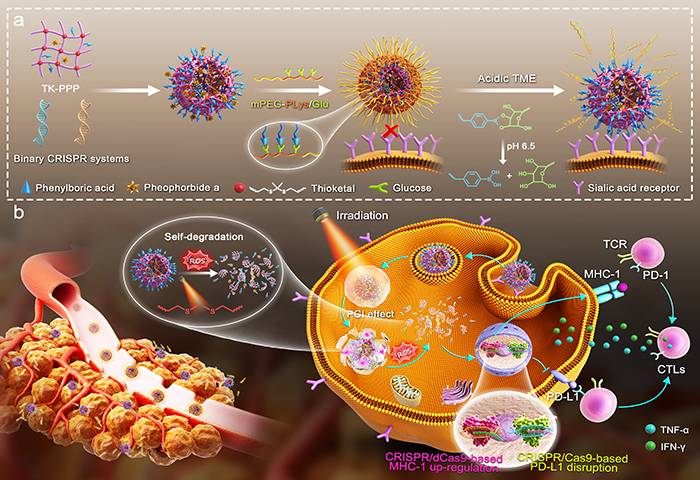
In response to the uncontrollable activation of the CRISPR gene editing system in normal physiological environments, a pH-photo responsive CRISPR nanodrug (DBCN) that can limit the specific activation of the CRISPR system at tumor sites was designed. This DBCN is made of a thioketal-cross-linked polyplex core and a tumor-microenvironment-responsive polyethylene glycol (PEG) shell, which can maintain stability during blood circulation while detaching a polymer shell to facilitate the cellular internalization of the CRISPR system after entering tumor tissues and ultimately activating gene editing under exogenous laser irradiation, thereby maximizing the therapeutic benefits and reducing potential safety concerns.
The paper shows that collaborative application of multiple CRISPR systems have been achieved for the first time. DBCN efficiently up-regulated the expression of the main histocompatibility complex class I (MHC-1) and corrected the programmed death ligand 1 (PD-L1) in tumors, thus initiating robust T cell-dependent antitumor immune responses to inhibit the growth of primary and distant tumors. In addition, DBCN further activated the specific anti-tumor immunological memory to significantly inhibited the recurrence and metastasis of malignant tumors.
Source: https://pubs.acs.org/doi/10.1021/acsnano.2c12107
